-
Publish Your Research/Review Articles in our High Quality Journal for just USD $99*+Taxes( *T&C Apply)
Offer Ends On
Ayse Oner* and Neslihan Sinim Kahraman
Corresponding Author: Ayse Oner, Acibadem Health Group, Acibadem Kayseri Hospital, 38040 Kayseri, Turkey.
Received: September 07, 2021 ; Revised: October 02, 2021 ; Accepted: October 05, 2021
Citation: Oner A & Kahraman NS. (2021) Comparison of Suprachoroidal and Subtenon Stem Cell Delivery Methods in Patients with Retinitis Pigmentosa. J Stem Cell Ther Res, 1(1): 1-8.
Copyrights: ©2021 Oner A & Kahraman NS. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Views & Citations
Likes & Shares
Abstract
Background: The aim of this study was to compare the efficacy and the safety of three different stem cell delivery methods in patients with retinitis pigmentosa (RP).
Methods: This prospective, single-center, clinical study enrolled 84 eyes of 84 patients who had a confirmed diagnosis of RP. The patients received 5 million umbilical cord derived mesenchymal stem cells (UC-MSCs) to the extraocular area with three different implantation techniques: 1: Standart suprachoroidal implantation 2: Modified suprachoroidal implantation 3: Subtenon implantation. Patients were evaluated on the first day, first month and sixth month postoperatively. Best corrected visual acuity (BCVA), anterior segment and fundus examinations, color photography, optical coherence tomography (OCT) and visual field (VF) tests were carried out at each visit. Fundus fluorescein angiography (FFA) and multifocal electroretinography (mfERG) recordings were performed before the treatment and at the end of the sixth month after treatment. Ocular and systemic adverse events related to the the surgical procedures were also noted.
Results: All patients completed the 6-month follow-up period. None of them had any serious systemic or ocular complications. After the treatment, we found statistically significant improvements in BCVA and VF-MD values in all groups when compared to baseline values (p<0.05). In the pairwise comparison of post-treatment values, there were statistically significant improvements in BCVA and VF-MD in group 2 when compared to group 1 and 3 (p
Conclusions: This study demonstrated beneficial effect of suprachoroidal and subtenon administration of UC-MSCs on BCVA, VF and mfERG measurements during the 6-month follow-up period. Decreasing the scleral thickness may enhance the effect of therapy via increasing the diffusion of growth factors secreted by stem cells.
Keywords: Stem cell therapy, Subtenon implantation, Suprachoroidal implantation, Retinitis pigmentosa, Umbilical cord derived mesenchymal stem cells, Visual function
INTRODUCTION
Retinitis pigmentosa (RP) is a heterogeneous group of hereditary retinal disorders characterized by progressive degeneration of photoreceptors. It primarily and severely affects the rods which causes night blindness and tunnel vision. In the late stage of the disease the involvement of the cones leads to loss of central vision and total blindness [1,2].
Although the disease is known to be genetical, the etiopathogenesis cannot be explained by genetics alone. Recent researches showed that there are other mechanisms including trophism, oxidation, inflammation, immune response, vascularization, and apoptosis [2].
The discovery of the trophic factors and their importance in promoting photoreceptor survival leads the efforts of developing cell preservation therapies. Mesenchymal stem cells (MSCs) are known to provide trophic support for neuroprotection and regeneration of damaged retinal cells [3-5]. Recent studies demonstrated that umbilical cord-derived MSCs (UC-MSCs) show significant paracrine and immunomodulatory features by producing trophic factors. Clinical studies also reported that UC-MSCs are effective in preventing retinal degeneration and rescuing photoreceptors [7-9].
Stem cell therapy is a new therapeutic option and there is not a guideline or a consensus for the surgeons to decide on which stem cell type and which way of application to offer in which patients. There are various ways of stem cell delivery to the eye in clinical studies including subretinal, intravitreal, suprachoroidal, subtenon and intravenous application routes.
The decision of the implantation method may depend on the target tissue and disease state [7-15]. However, subretinal and intravitreal injections are associated with some posterior and anterior segment complications [10,11,15]. Using periocular routes and implanting stem cells to the suprachoroidal and subtenon space is reported to be safe and effective in recent publications [7,8,12]. The biggest study in the literature including 124 eyes of 82 RP patients showed that implantation of UC-MSCs to the suprachoroidal area had no serious systemic or ocular complications. This study also showed statistically significant improvements in visual acuity, visual field (VF) and multifocal electroretinography (mfERG) tests during the study period [8].
The suprachoroidal space is a potential space between the sclera and choroid that traverses the circumference of the posterior segment of the eye. This space is an attractive site for drug delivery because it targets the choroid retinal pigment epithelium and retina with high bioavailability [12].
This study is conducted to compare the effects of suprachoroidal and subtenon stem cell delivery methods and to provide further knowledge for the clinicians in the management of patients. The study will also help to understand the advantages and disadvantages of different implantation routes for stem cell therapy.
METHODS
Study design and setting
This prospective open label clinical study was conducted to compare the efficacy and safety of suprachoroidal and subtenon stem cell implantation methods in RP patients in the ophthalmology department of our hospital between 01.03.2018 and 01.11.2020. The study was performed in accordance with the Declaration of Helsinki, after obtaining the approval of the Ethics Committee of the University (2017/480, 13.10.2017) and the approval by the Review Board of Stem Cell Applications of the Ministry of Health (56733164/203) according to the regulations in our country. Written informed consent was obtained from all participants of the study.
Patients
After receiving a complete medical history, the patients were evaluated for eligibility according to the inclusion and exclusion criteria. The inclusion criteria of the study were: (1) 18 years and older age (2) clinical diagnosis of RP confirmed by ophthalmological tests (3) having best corrected visual acuity (BCVA) of <20/40 (4) having various degrees of VF loss. The exclusion criteria of the study were: (1) having previous ocular surgery other than cataract extraction (2) presence of ocular media opacities that would make the image quality not sufficient for ocular imaging or effect mfERG or VF evaluation, (3) having coexisting ocular disease [i.e., retinal pathology, uveitis, strabismus, nystagmus] (4) having systemic disease that would affect the results and (5) having the habit of smoking. Patients were randomly divided into three groups. Group 1 included patients who received surgery with standard suprachoroidal implantation. In Group 2 surgery was performed with a modified suprachoroidal implantation technique. In Group 3, stem cells were implanted with a subtenon method.
Variables and outcomes
After recording the demographic variables, all patients received a detailed ophthalmic examination including BCVA and intraocular pressure measurements, anterior segment evaluation with slit-lamp biomicroscopy, color fundus photography, optical coherence tomography (OCT), fundus fluorescein angiography (FFA), VF and mfERG tests.
The BCVA, VF and mfERG outputs were three primary outcomes of our study. VF examination was performed by Humphrey VF analyzer device (Carl Zeiss Meditec AG Germany), program 30-2 was used for testing of each eye. BCVA was recorded with a Snellen chart at a distance of 3 meters. MfERG was recorded on mfERG Vision monitor (Metrovision, France). The mfERG readings were recorded from each eye according to the International Society for Clinical Electrophysiology of Vision (ISCEV) guidelines [16].
During the mfERG evaluations, a matrix of 61 hexagons of the individual mfERG responses were generated, and these hexagons were grouped into five concentric rings (<2°, 2-5°, 5-10°, 10-15° and >15°) centered on the fovea. We recorded the average amplitude of the first positive wave (P1) in these five rings.
The study also aimed to report the adverse events related to the stem cell implantation and surgical procedures.
The stem cell preparation: The stem cells were prepared from umbilical cord tissue according to the procedure described elsewhere. 8 A concentration of 5x106 cells in isotonic solution containing 1% human serum albumin were transferred in vials with the temperature-controlled bag within 12 h, and the product was used within 24 h.
Surgical procedures
An experienced surgeon (AO) performed all surgeries. All operations were carried out under sterile conditions with subtenon bupivacaine anesthesia.
Standard suprachoroidal implantation: We performed a surgical technique defined as the Limoli retinal restoration technique (LRRT), described by Limoli [12]. The technique was also used by our group in our previous studies [8,17,18]. The details of the surgery were as follows: The globe was deviated to the superonasal quadrant and conjunctiva was dissected at the inferotemporal quadrant at 8 mm from the limbus. A deep scleral flap of about 5 X 5 mm was opened by a radial hinge at the inferotemporal quadrant. The flap was deep enough to allow viewing of the color of the choroid. A flap from the orbital fat was extracted from a gap above the inferior oblique muscle. This fat tissue was laid on the scleral bed and sutured with 6/0 vicryl at the proximal edge. The scleral flap was then sutured above the fat pedicle. The remaining space between the autologous fat graft, choroid, and scleral flaps was filled with 5x106 UCMSCs. The conjunctiva was sutured with 8/0 vicryl (Figure 1).
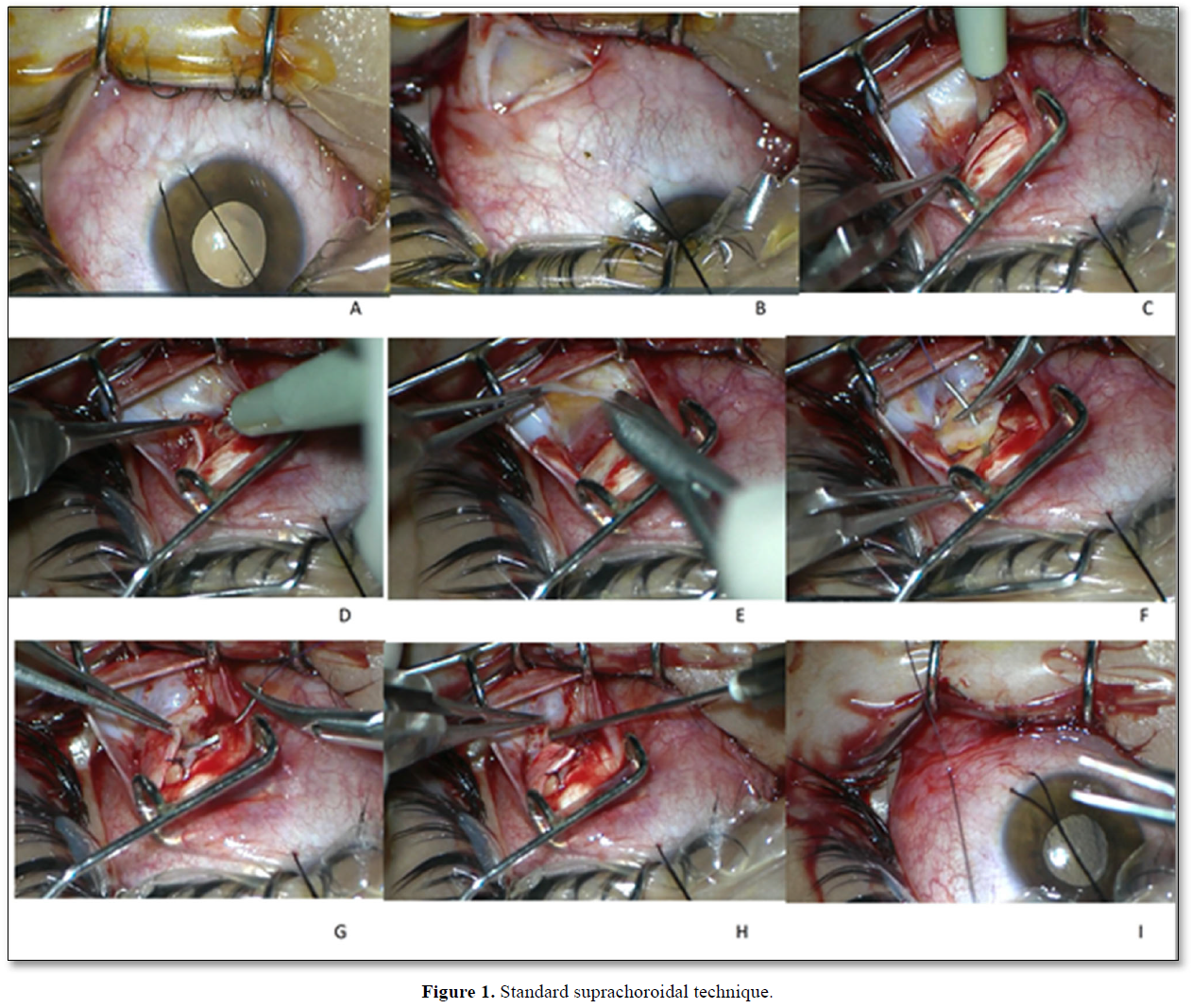
This figure showed the details of the surgery. A. The globe was deviated to the superonasal quadrant with a deviation suture. B. The conjunctiva and tenon capsule were dissected with a tenotomy scissor at the inferotemporal quadrant at 8 mm from the limbus. C. The conjunctiva and tenon capsule were separated from the surgical area with a pediatric eye speculum. A deep scleral flap of about 5 X 5 mm was opened by a radial hinge at the inferotemporal quadrant. D. The sclerectomy was deep enough to allow viewing of the color of the choroid. The distance between the grafted stem cells and choroid was reduced by deep sclerectomy to enhance the paracrine effect of transplanted stem cells. E. A flap from the orbital fat was extracted from a gap above the inferior oblique muscle. F. This fat tissue was laid on the scleral bed and sutured with 6/0 vicryl suture at the proximal edge of the scleral bed. G. The scleral flap was then sutured above the fat pedicle. H. The remaining space between the autologous fat graft, choroid, and scleral flaps was filled with 1 cc of 5x106 UCMSCs. The cells were injected with a 22-gauge needle. I. The conjunctiva was sutured with 8/0 vicryl.
Modified suprachoroidal implantation: This technique included all of the steps of the standard technique. A modification was made in the preparation of the scleral fleb. To decrease the scleral thickness and to enhance the diffusion of stem cells and secreted growth factors we create a second scleral fleb smaller than the first one (2x2 mm) in the scleral bed. The sclera was dissected with extreme attention to avoid hemorrhage until the choroid tissue is exposed and 2x2 mm choroid tissue has a direct contact with the implanted stem cells (Figure 2).

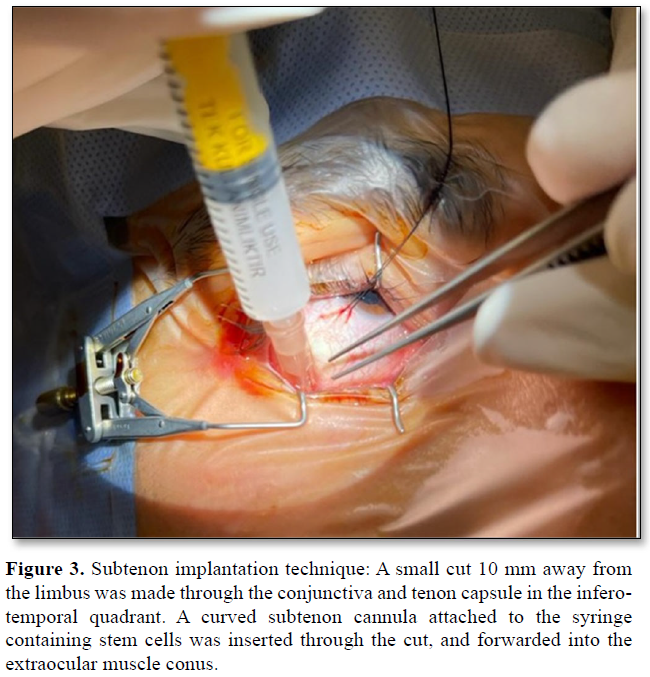
Subtenon implantation: A small cut 10 mm away from the limbus was made through the conjunctiva and tenon capsule in the infero-temporal quadrant. A curved subtenon cannula attached to the syringe containing stem cells was inserted through the cut, and forwarded into the extraocular muscle conus. Stem cells was then injected through the cannula and insertion site was pressed with a sponge to prevent the leakage. Subsequently, the conjunctiva and tenon were sutured with an 8/0 vicryl suture (Figure 3).
Postoperative follow-up
In all groups topical antibiotic and steroid drops were administered four times a day for one month after surgery. Ophthalmic evaluations, including BCVA, anterior and posterior segment examination, color fundus photographs and OCT were performed before surgery, and at the postoperative first day, 1st month, 3rd month and 6th month. We also performed VF at 1st, 3rd and 6th months, FFA and mfERG at 6th month postoperatively. All patients were evaluated for adverse events of the surgical procedures during the study period.
Statistical analysis
Statistical analyses were conducted using SPSS version 20 statistical package program (IBM Corp. in Armonk, NY). Descriptive data are presented as median with interquartile range for non-normally distributed numerical variables. Shapiro-Wilk and Kolmogorov-Smirnov tests were used to evaluate the distribution of the numeric data. The Related-Samples Friedman’s Two-Way Analysis of Variance by Ranks test was used for comparing the VF and BCVA measurements and the Wilcoxon signed-rank test with a Bonferroni correction was conducted for post-hoc pairwise comparison. MfERG measurements were compared using the Wilcoxon signed-rank test. p
RESULTS
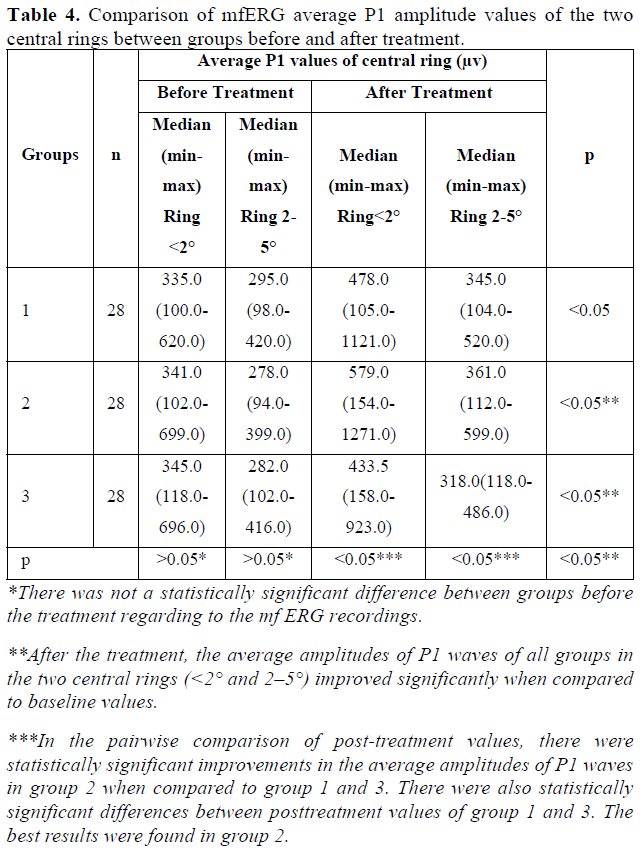
All patients completed 6-month follow-up period. The median age and the gender ratio of the groups did not differ significantly (p>0.05) (Table 1). There was not a statistically significant difference between groups before the treatment regarding to the BCVA, VF mean deviation (VF-MD) value and mf ERG recordings (p>0.05) (Tables 2-4).
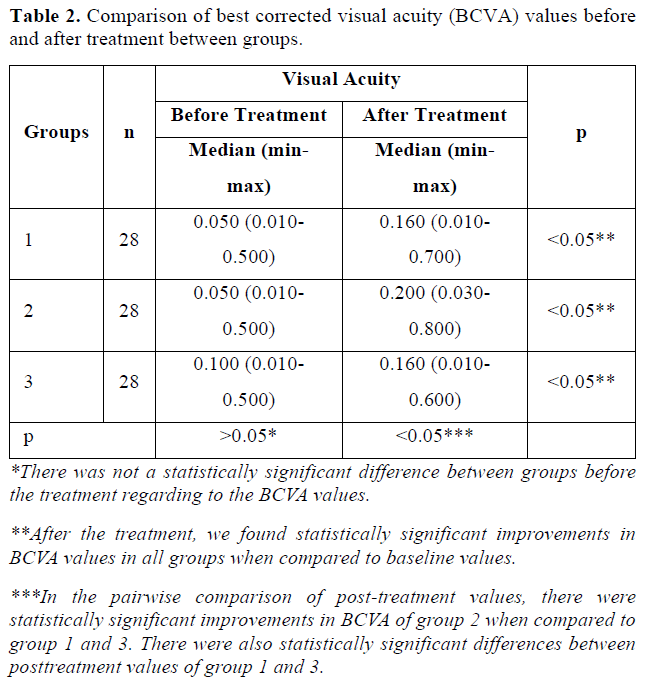
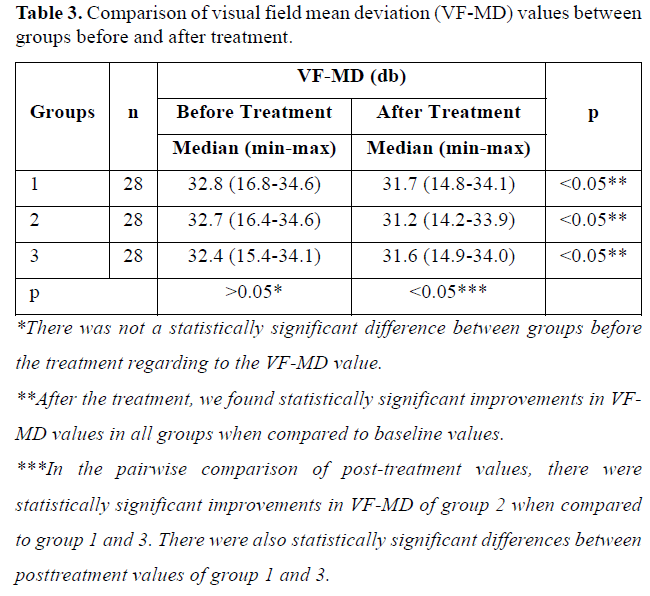
After the treatment, we found statistically significant improvements in BCVA and VF-MD values in all groups when compared to baseline values (p<0.05) (Tables 2 & 3). In the pairwise comparison of post-treatment values, there were statistically significant improvements in BCVA and VF-MD in group 2 when compared to group 1 and 3 (p(Tables 2 & 3).
Regarding to the mfERG test, the average amplitudes of P1 waves in the two central rings (<2° and 2-5°) improved significantly at the postoperative 6th month in all groups when compared to baseline values (p<0.05). The amplitudes of the peripheral three rings (5-10˚, 10-15° and >15° rings) were not statistically significant (p>0.05). The average implicit times of P1 waves in all rings did not differ after stem cell implantation (p>0.05) (Table 4).
All patients who underwent stem cell treatment revealed no morphological changes in OCT scans of both eyes of the patients. The mean central macular thickness measurements of the all-treated eyes did not show any significant changes after treatment (p>0.05). None of the patients experienced serious ocular adverse events during 6-month follow-up period.
DISCUSSION
In recent years, there have been significant developments about stem cells therapies for retinal diseases. There are various ways for stem cell delivery to the eye in clinical studies consisting of subretinal, intravitreal, suprachoroidal, subtenon and intravenous application routes [9-15]. However, subretinal and intravitreal injections are associated with some posterior and anterior segment complications [10,11,15]. In a phase I study including subretinal injection of stem cells in 14 advanced stage RP patients, the researchers reported development of choroidal neovascular membrane in one patient and epiretinal membrane with peripheral tractional retinal detachment in six patients. One of the patients experienced mild band keratopathy six months after the treatment and another patient had retrolental fibrous tissue at 1-year follow-up examination. Those complications were thought to be secondary to the inadvertent preretinal injection of cells or reflux of transplanted cells from the subretinal space. Underlying vitreous abnormalities existing in patients with advanced stage RP might cause difficulties in removing the whole posterior vitreous and the unwanted proliferation of scattered epiretinal MSCs were thought to induce these complications. With a modified technique the authors paid extreme attention to shave the peripheral vitreous, to visualize the residual posterior hyaloid remnants or preretinal membranes and remove them. They also rinse the scattered MSCs on the epiretinal area. These modifications prevented the development of complications in the remaining patients of the study and three patients experienced visual acuity improvement. Authors reported that subretinal implantation of MSCs may have some adverse effects and the patients should be followed up carefully [10,11].
In another study the authors reported extensive preretinal and vitreous fibrosis which caused tractional retinal detachment and vision loss in one of the three patients which received intravitreal stem cell injections. Ocular examination of this patient showed vision of no light perception, mature cataract, iris neovascularization, ocular hypotony and shallow anterior chamber at 1-year follow-up. The authors investigated these cells in animals and they observed retinal glial responses to transplanted cells [15].
Suprachoroidal, subtenon or peribulbar administration methods are reported to have no serious ocular complications [7,12,13,17,18]. In the largest stem cell study in the literature including 124 eyes of 82 RP patients, UC-MSC implantation was performed to the suprachoroidal space and no serious ocular adverse effects were reported. Suprachoroidal delivery method being near the choroid also has the advantage of allowing the produced growth factors to enter the choroidal flow and managing the consent growth factor secretion to the choroidal and retinal tissues. This method has been first introduced by Limoli and his colleagues and was proven to be safe with no complications [12,18].
Subtenon implantation method of stem cells are also reported to be safe in recent studies. In a phase 3 study by Ozmert et al. Wharton’s jelly derived MSCs were injected to the subtenon space in 32 RP patients. They reported a significant improvement in the mean BCVA of the patients which increased from 70.5 letters to 80.6 letters at the 6-month follow-up. They also found significant improvements in VF, mfERG, full-field flicker ERG tests and mean outer retinal thickness measurements. No ocular or systemic adverse events were observed related to the Wharton’s jelly derived MSC implantation during the follow-up period in this study [7].
It is known that, after periocular implantation like subconjunctival, subtenon or retrobulbar, the stem cells directly contact with the sclera and the molecules secreted by the cells are thought to pass through the sclera mainly via passive diffusion. The delivery of the molecules to the retina via this route is affected by various physiological barriers, which include the scleral thickness, choroidal blood circulation and blood-retina barriers [19-21].
Experimental studies showed that the permeability coefficient of sclera has a significant negative relationship with scleral thickness [22]. As the permeability of sclera increases significantly with decreasing thickness, the thinnest equatorial part of the sclera may be the ideal position for surgeries or subtenon injections targeting choroid and retina [23,24].
In this study, the implantation area was around the equatorial area in the three surgical techniques. Decreasing the thickness of the sclera by creating a scleral fleb in the suprachoroidal methods may enhance the diffusion and bioavailability of the molecules to the retina. Obtaining the best results with the modified suprachoroidal technique may be evidence for the thickness theory.
External methods for suprachoroidal implantation only require penetration of the sclera and can be achieved via a full-thickness scleral incision. Identification of the scleral-choroidal junction requires slow and delicate dissection so as not to inadvertently cut through the choroid and retina. In the modified suprachoroidal method, the authors dissected the second fleb with extreme caution to avoid complication and there were no complications related to the surgical technique in the study patients.
In RP patients the choroid tissue was found to be thinner than normal patients in previous studies [25]. Besides the decreased thickness, the choroid has numerous fenestrations, pinocytotic vesicles, thus allowing for relatively easy movement of substances [26]. We believe that these changes in the choroidal tissue in RP patients enhances the diffusion of the molecules to the retina.
There are some studies in the literature comparing different routes of drug delivery to the eye. An experimental study investigating exposure of sodium fluorescein (NaF) to the choroid-retina region showed that delivery is greater with suprachoroidal injection when compared to intravitreal and transscleral routes. Peak NaF concentration in choroid-retina was 36-fold and 25-fold higher after suprachoroidal injection when compared to posterior subconjunctival and intravitreal injections, respectively. In this rat model suprachoroidal injections resulted in the highest bioavailability [27].
In another experimental study triamcinolone acetonide was administered to each rabbit eye by suprachoroidal and intravitreal injections. Results indicated a 12-fold greater concentration in the sclera/choroid/ retina with suprachoroidal versus intravitreal administration [28-29].
As a result, the suprachoroidal space is receiving increasing attention, and its potential role in the diagnosis and treatment of various retinal conditions is being recognized. It is also emerging as a target for drug delivery and surgical procedures.
CONCLUSION
RP is a genetic disorder that can cause total blindness with a degeneration process. With respect to the clinical experiences, cell mediated therapy based on the use of growth factors seems remarkable because they can improve the electrical cell response for a certain period of time. The follow-up period in our study is 6 months and it is not enough to determine how long the obtained improvements in visual function will persist, which treatment method will result better or a retreatment will be required. Long term follow-up will be needed to evaluate the durability of the treatment.
ACKNOWLEDGMENTS
We thank the participants of the study. We would like to thank Prof Dr. Ercument Ovalı and the staff members of Acibadem Lab cell for providing the stem cells. We also thank to the staff members of Acibadem Kayseri Hospital for their contribution to the study tests.
FUNDING
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
AUTHORS' CONTRİBUTİONS
NS: Patient follow-up, data collection, manuscript preparation.
AO: Study design, patient selection and follow-up, surgical intervention, data collection, manuscript preparation.
All authors read and approved the manuscript.
REFERENCES
No Files Found
Share Your Publication :